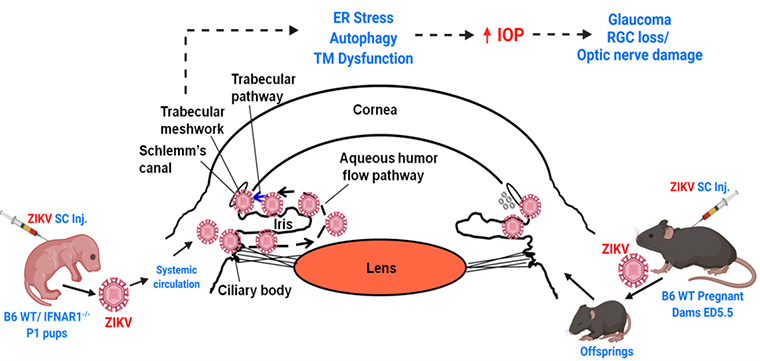
Zika virus pathogenesis in ocular infection
For the past 15 years, a new infectious disease has been discovered every year, with an outbreak reported on nearly every continent. One recent Zika virus (ZIKV) epidemic affected ~2 million people in the Americas including the United States. Although ZIKV was discovered in 1947, it gained global attention as an important pathogen in 2015 when it emerged as an epidemic and caused not only neurological complications but also severe ocular abnormalities and visual impairment. Newborns/ infants born from a mother infected with ZIKV during pregnancy exhibited severe ocular complications such as chorioretinal atrophy, hypoplasia, focal pigmented mottling, optic nerve atrophy/anomalies, severe retinal vessel attenuation, and macular pigmentation alterations. More recently, clinical cases from several parts of the world have linked ZIKV infection to congenital glaucoma in infants. As such, glaucoma is the second-leading cause of irreversible blindness worldwide and is primarily considered a genetic and age-related disease that has not been reported among infants exposed to infection during gestation, until recent ZIKV epidemics. Flaviviruses, including ZIKV, are now globally distributed and infect up to 400 million people annually and remain the leading cause of global health problems, but to date, their ocular complications remain underreported. Unlike other flaviviruses, ZIKV has been reported to cause congenital glaucoma, and prior flavivirus infections have been shown to potentiate the ZIKV induced glaucomatous pathology due to antibody-dependent enhancement. Considering the fact that ZIKV endemically circulating in more than 84 countries worldwide, the risk is global, and it is imperative to study the pathobiological mechanisms of ZIKV-induced glaucoma and to identify potential targets for future therapeutic intervention.
Recently, using mouse models of ZIKV infection, we discovered that ZIKV can cause an increase in intraocular pressure (IOP), retinal ganglion cell (RGC) loss, and damage to the optic nerve (PMCID: PMC6506617), a hallmark of glaucoma. Under this project, using both in vitro and in vivo models (neonatal and pregnancy models of infections) we are investigating the molecular mechanism of the disease and testing pathway-based therapies and novel anti-viral molecules for its treatment.
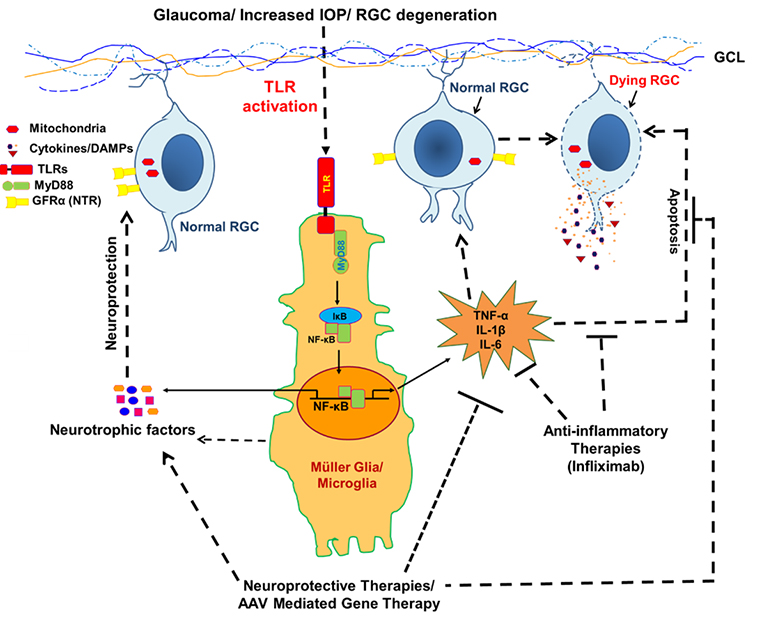
Role of innate immunity in glaucoma pathobiology and discovery of neuroprotective therapies:
Glaucoma is a progressive optic neuropathy characterized by the gradual degeneration of RGCs and their axons with accompanying loss of the visual field over time. Currently, glaucoma affecting around 70 million people and is estimated to increase up to 111.8 million by 2040. Although the cause of glaucoma is not clearly established, multiple risk factors including race, age, and elevated IOP can contribute to glaucoma. Previously, it was thought that glaucoma was caused only by increased IOP, however, glaucomatous RGC and its axons loss have been seen in individuals even with normal IOP, and the patients whose IOP is controlled by IOP lowering drugs. In the recent past, several other potential contributors believed to play role in the development of glaucomatous neuropathies that are neuroinflammation, alteration of neurotrophins (NTs) signaling, mitochondrial dysfunction, protein misfolding, oxidative stress, metabolic abnormalities, and blood flow disturbances. Among these pathogenic mechanisms proposed for neurodegeneration, neuroinflammation is of increasing interest. Recently we discovered the activation of various inflammatory/inflammasome pathways in our glaucoma model. Similarly, using aqueous samples from glaucoma patients we discovered alteration of many key metabolites in these patients.
Under this project, using a microbead-induced ocular hypertension model of glaucoma we will be investigating the molecular mechanisms involving host immune components, neuroinflammatory signaling, cellular senescence, and cell death pathways in the pathogenesis of glaucoma. Using cutting-edge and high throughput “omics” technologies (such as transcriptomics, metabolomics, and proteomics) in glaucoma patients samples we will be investigating the immuno-metabolic cross-talk and looking for metabolic markers that could be either used as a disease biomarker or tested as a therapeutic agent. We are using an AAV-mediated gene therapy approach to test few neurotrophic agents for neuroprotective therapies for RGC protection from neuroinflammatory damage.
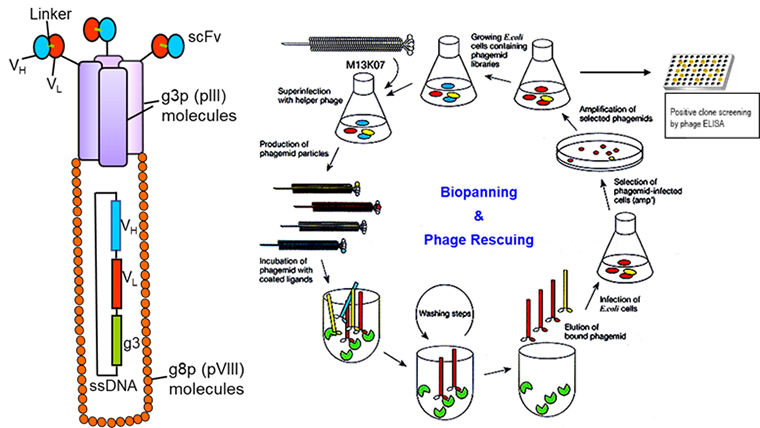
Development of recombinant protein/ antibody-based therapeutics for SARS-CoV2 and Staphyloccoal superantigens.
Vaccine design and development platforms against infectious agents are classified into many categories that include nucleic acid-based vaccines (mRNA or DNA), viral vector-based vaccines (such as adenovirus), inactivated or live attenuated vaccines, and recombinant protein or peptide-based vaccines. Recombinant protein or peptide-based vaccines have been used in the medical setting for nearly a century and represent an inexpensive and versatile tool for large-scale immunization. Many recombinant antibodies are in use for the treatment of various diseases including cancer and infectious diseases. Phage display is a molecular technique that allows the presentation of large protein and peptide libraries on the surface of filamentous phage and permits the selection of such proteins, peptides, and antibodies with high affinity and specificity for almost any target. Using a phage display technology we have developed a high-affinity scFv antibody against a staphylococcal superantigen SEB (PMCID: PMC3008221). During the current COVID-19 pandemic, many therapeutic antibodies were developed which are currently in various stages of preclinical and clinical trials. Around 85% of antibodies against SARS-CoV2 were developed targeting the Spike (S) protein, primarily focused on targeting receptor-binding domain (RBD) to block the viral entry. Our long-term goal for this project is to develop antibody-based therapeutics/vaccines against infectious agents. We will be using a site-directed mutagenesis approach and phage display technology to develop protein/ antibody-based therapeutics development against SARS-CoV2 and staphylococcal superantigens.